HARD
JEE Advanced
IMPORTANT
Earn 100
The correct statement(s) regarding,
(i)
(ii)
(iii) and
(iv) is /are
(a)The number of bonds in (ii) and (iii) together is two.
(b)The number of lone pairs of electrons on Cl in (ii) and (iii) together is three.
(c)The hybridization of Cl in (iv) is
(d)Amongst (i) to (iv), the strongest acid (i)
56.41% studentsanswered this correctly

Important Questions on The p-Block Elements
HARD
JEE Advanced
IMPORTANT
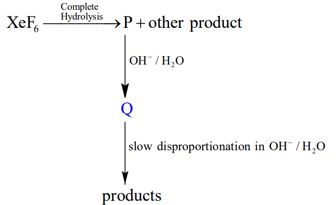
Under ambient conditions, the total number of gases released as products in the final step of the reaction scheme shown below is
EASY
JEE Advanced
IMPORTANT
The product formed in the reaction of with white phosphorous is
HARD
JEE Advanced
IMPORTANT
The correct statement(s) about O3 is(are)
HARD
JEE Advanced
IMPORTANT
With respect to hypochlorite, chlorate and perchlorate ions, choose the correct statement(s).
MEDIUM
JEE Advanced
IMPORTANT
The green colour produced in the borax bead test of a chromium(III) salt is due to -
MEDIUM
JEE Advanced
IMPORTANT
Among and the number of molecules containing covalent bond between two atoms of the same kind is __________
MEDIUM
JEE Advanced
IMPORTANT
At the reaction of with produces a xenon compound The total number of lone pair(s) of electrons present on the whole molecule of is __________
MEDIUM
JEE Advanced
IMPORTANT
The compound(s) which generate(s) gas upon thermal decomposition below is (are)
