MEDIUM
NEET
IMPORTANT
Earn 100
The correct statement regarding the basicity of aryl amines is:
(a)Aryl amines are generally less basic than alkyl amines because the nitrogen lone-pair electrons are delocalized by interaction with the aromatic ring electron system.
(b)Aryl amines are generally more basic than alkyl amines because the nitrogen lone-pair electrons are not delocalized by interaction with the aromatic ring electron system.
(c)Aryl amines are generally more basic than alkyl amines because of aryl group.
(d)Aryl amines are generally more basic than alkyl amines, because the nitrogen atom in aryl amines is sp-hybridized.
90% studentsanswered this correctly

Important Questions on Organic Chemistry- Some Basic Principles and Techniques
MEDIUM
NEET
IMPORTANT
The correct statement regarding a carbonyl compound with a hydrogen atom on its alpha carbon is.
EASY
NEET
IMPORTANT
Which among the given molecules can exhibit tautomerism?
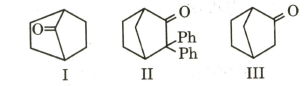
MEDIUM
NEET
IMPORTANT
The correct order of strengths of the carboxylic acids:

EASY
NEET
IMPORTANT
In pyrrole
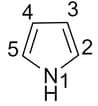
The electron density is maximum on
EASY
NEET
IMPORTANT
The correct increasing order of basic strength for the following compounds is :
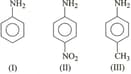
EASY
NEET
IMPORTANT
The correct statement regarding electrophile is:
EASY
NEET
IMPORTANT
Which one is the most acidic compound?
MEDIUM
NEET
IMPORTANT
Among the following alkenes: -butene(, cis- -butene, trans--butene, the decreasing order of stability is
