The criteria for the spontaneity of a process are

Important Questions on Thermodynamics
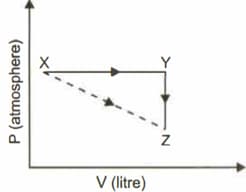
For an ideal gas, consider only work in going from an initial state to the final state . The final state can be reached by either of the two paths shown in the figure. Which of the following choice(s) is (are) correct ? [take as change in entropy and as work done].
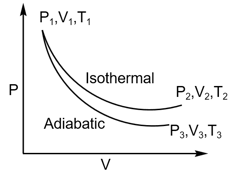
The reversible expansion of an ideal gas under adiabatic and isothermal conditions is shown in the figure. Which of the following statement(s) is (are) correct?
An ideal gas in a thermally insulated vessel at internal pressure =, volume = and absolute temperature= expands irreversibly against zero external pressure, as shown in the diagram. The final internal pressure, volume and absolute temperature of gas are, and respectively.
For this expansion which of the following conditions holds correct?