MEDIUM
Earn 100
The crystal filed theory is successful in explaining which of the following?
I. Ligands as point charges;
II. Formation and structures of complexes.
III. colour;
IV. Magnetic properties:
V . covalent character of metal-ligand bonding.
(a) only
(b) only
(c) only
(d) only
41.22% studentsanswered this correctly
Important Questions on Coordination Compounds
MEDIUM
MEDIUM
MEDIUM
MEDIUM
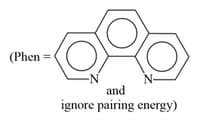
HARD
EASY
HARD
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
(Atomic No. = Ti : 22, Cr : 24 and Mo : 42)
MEDIUM
MEDIUM

