HARD
Earn 100
The curve in the figure shows the variation of the during the course of the titration of a weak acid , with a strong base (). At which point in the titration curve is the concentration of the acid equal to that of its conjugate base?
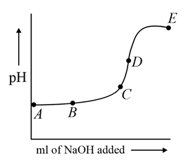
(a)Point D.
(b)Point E.
(c)Point C.
(d)Point B.
24.1% studentsanswered this correctly
Important Questions on Transfer
MEDIUM
Statement I : In the titration between strong acid and weak base methyl orange is suitable as an indicator.
Statement II : For titration of acetic acid with $\mathrm{NaOH}$ phenolphthalein is not a suitable indicator.
In the light of the above statements, choose the most appropriate answer from the options given below:
HARD
HARD
The pH of the solution after Expt. 2 is