The decomposition of compounds due to light is called photolysis.
Important Questions on Chemical reactions and Equations
Calcium oxide reacts vigorously with water to produce slaked lime.
This reaction can be classified as:
(A) Combination reaction (B) Exothermic reaction
(C) Endothermic reaction (D) Oxidation reaction
Which of the following is the correct answer?
When hydrogen sulphide gas is passed through a blue solution of copper sulphate, a black precipitate of copper sulphide is obtained and the sulphuric acid so formed remains in the solution. The reaction is an example of a :
Answer the following:
In the chemical reaction given below:
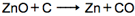
Name the substance which is reduced?
In a double displacement reaction such as the reaction between sodium sulphate solution and barium chloride solution:
(A) exchange of atoms takes place.
(B) exchange of ions takes place.
(C) a precipitate is produced.
(D) an insoluble salt is produced.
The correct option is:
Answer the following:
In the chemical reaction given below:
Identify the oxidising agent.
Which of the following reaction does not takes place? State the reason.
(i)
(ii)
Which reactant is oxidised in the following chemical reaction?
Write the balanced chemical equation for the following chemical reaction.
(a) Identify the type of reaction and the gas X.
(b) Write balanced chemical equation of the reaction.
(c) Write the range of aqueous solution of the gas X.

