The decomposition of hydrogen peroxide in an aqueous solution is a first order reaction. It can be studied by titrating quickly portions of reactions mixture at various times from the of reaction against a standard solution of . Volume of solution used in each case is proportional to the remaining concentration of
From the following data calculate the rate constant of the reaction.
Time (seconds)
solution used

Important Questions on Chemical Kinetics
The thermal decomposition of occurs in the following steps.
Step-
Step-
Suggest the rate expression.
The reaction , obeys the following mechanism.
(i)
(ii)
Suggest the rate expression.
Given the following steps in the mechanism for a chemical reaction:
(fast)
(Slow)
(fast)
At any time is directly proportional to
(a) What is the stoichiometric equation for the reaction?
(b) Which species, if any, are catalysts in this reaction ?
(c) Which species, if any, are intermediates in this reaction?
(d) Write the rate law for the rate-determining step.
(e) Write the rate law for this reaction.
(f) What is overall order of the reaction ?
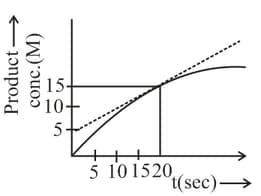
Rate of formation of product at seconds is:
