MEDIUM
Earn 100
The decreasing order of bond enthalpies of the following alkyl halides is:
(i)
(ii)
(iii)
(iv)
(a)
(b)
(c)
(d)
72.26% studentsanswered this correctly
Important Questions on Haloalkanes and Haloarenes(OoS)
HARD
MEDIUM
EASY
(A)
(B)
(C)
(D)
MEDIUM
MEDIUM
HARD
MEDIUM
MEDIUM
MEDIUM
EASY
This reaction will be the fastest in
EASY
EASY
EASY
MEDIUM
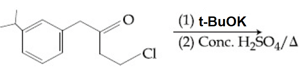
EASY
EASY
MEDIUM
MEDIUM
EASY
MEDIUM

