EASY
Earn 100
The density of glycerol is higher than propanol due to:
(a)More number of covalent bonds
(b)Ionic bonding
(c)Hydrogen bonding
(d)Van der Waal's attraction
50% studentsanswered this correctly
Important Questions on Alcohols, Phenols and Ethers
EASY
EASY
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
Given below are two statements : One is labelled as and the other is labelled as .
Butan––ol has higher boiling point than ethoxyethane.
Extensive hydrogen bonding leads to stronger association of molecules.
In the light of the above statements, choose the correct answer from the options given below :
MEDIUM
EASY
MEDIUM
MEDIUM
Arrange the following alcohols in order of their increasing boiling points.
Pentan--ol Butan--ol Butan-ol Propane--ol Ethanol
I II III IV V
MEDIUM
EASY
Give the reasons :
Boiling points of alcohols are higher than ethers.
MEDIUM
MEDIUM
MEDIUM
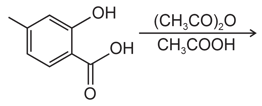
EASY
EASY
MEDIUM

