MEDIUM
Earn 100
The descending order of acidity for the following carboxylic acid is-
(A)
(B)
(C)
(D)
(E)
Choose the correct answer from the options given below:
(a)
(b)
(c)
(d)
83.33% studentsanswered this correctly
Important Questions on Some Basic Principles of Organic Chemistry
HARD
The correct order of acidity for the following compounds is :
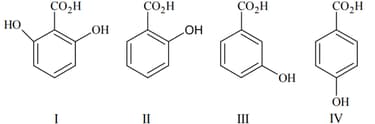
MEDIUM
The correct order for acid strength of compounds and is as follows:
MEDIUM
Arrange the following labelled hydrogens in decreasing order of acidity:
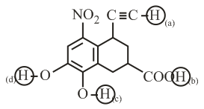
MEDIUM
Find the correct order of acid strengths of the following compounds
MEDIUM
The compound that does NOT liberate CO2, on treatment with aqueous sodium bicarbonate solution, is
EASY
The increasing order of acidic strength among the following compounds is
. Benzoic acid
. nitrobenzoic acid
. dinitrobenzoic acid
. methoxybenzoic acid
MEDIUM
Which one of the following compounds possesses the most acidic hydrogen?
MEDIUM
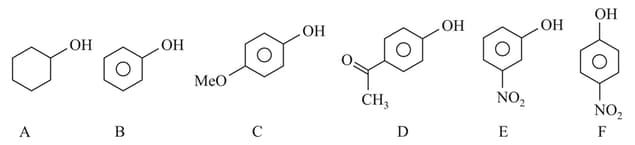
The correct order of acid character of the following compounds is :
EASY
Among formic acid, acetic acid, propanoic acid and phenol, the strongest acid in water is
HARD
An organic compound showing the following solubility profile is:
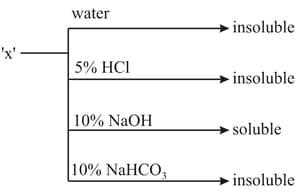
EASY
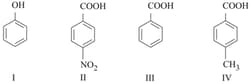
The correct order of acidity for the following compounds is :
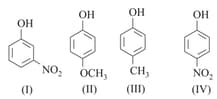
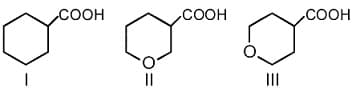
HARD
The correct order of strengths of carboxylic acids is
EASY
Which order of arrangement is correct in terms of the strength of the acid?
MEDIUM
The increasing order of the acidity of the -hydrogen of the following compounds is:
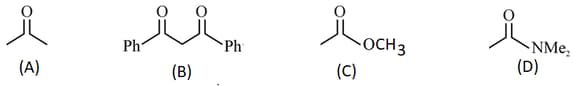
MEDIUM
The acidity of
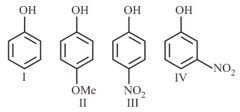
Follows the order
EASY
Arrange Benzene, n-Hexane and Ethyne in decreasing order of their acidic behaviour
MEDIUM
What will be the CORRECT decreasing order of acid strength of the hydroxybenzoic acids ? (Symbols and notations carry their usual meanings)
EASY
The correct order of acid strength of the following carboxylic acids is -
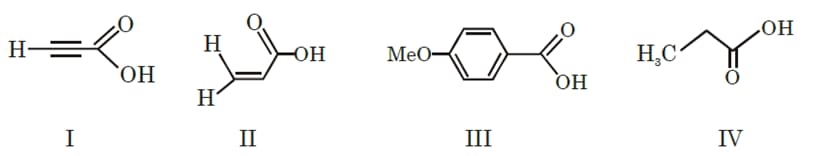
MEDIUM
The correct order of acidity of the following compounds is

MEDIUM
Among the following, which is least acidic?

