EASY
NEET
IMPORTANT
Earn 100
The diagram below shows the energy levels for an electron in a certain atom. Which transition shown represents the emission of a photon with the most energy?
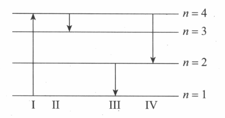

(a)III
(b)IV
(c)I
(d)II
50% studentsanswered this correctly

Important Questions on Atoms and Nuclei
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
