HARD
JEE Main
IMPORTANT
Earn 100
The diagram given below depicts the boiling point as the function of composition of the mixture of and . Which of the following statement about the diagram is/are true?
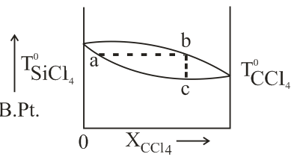

(a)The point represents the composition of solution and the point b that of the vapor in equilibrium.
(b)The proportion of in the solution is smaller than that in the vapor in equilibrium.
(c) represents the condensation of the vapor.
(d)The point c represents the composition of solution and the point b that of the vapor in equilibrium.
50% studentsanswered this correctly

Important Questions on Solutions
MEDIUM
JEE Main
IMPORTANT
Explain why on the addition of mole of to of water, the boiling point of water increases, while the addition of a mole of methyl alcohol to of water, decreases its boiling point?
EASY
JEE Main
IMPORTANT
What is the reason that the vapour pressure of an aqueous solution of glucose is lower than that of water?
EASY
JEE Main
IMPORTANT
each of two non-electrolyte solutes and (molar mass of is greater that of ) are dissolved separately in each of the same solvent. Which one will show a greater elevation in the boiling point?
HARD
JEE Main
IMPORTANT
An aqueous solution containing of a non-volatile compound, having the stoichiometric composition in of water, boils at under atm pressure. What is the molecular formula of the given compound?
HARD
JEE Main
IMPORTANT
A solution contains of a non-volatile solute in exactly of water, it has a vapour pressure of at Further, of water is then added to the solution, the new vapour pressure becomes of at . Calculate the molar mass of the solute.
HARD
JEE Main
IMPORTANT
A solution contains of a non-volatile solute in exactly of water, its vapour pressure is of at Further, of water is then added to the solution, the new vapour pressure becomes of at . Calculate the vapour pressure of water at .
HARD
JEE Main
IMPORTANT
What mass of the non-volatile solute urea needs to be dissolved in of water, in order to decrease the vapour pressure of water by ? What will be the molality of the solution?
MEDIUM
JEE Main
IMPORTANT
Molal elevation constant of a liquid is
