The diagram given below is to prepare iron [III] chloride in the laboratory:
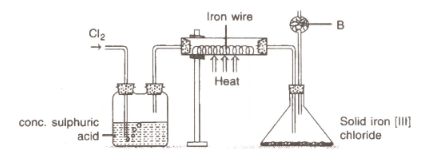
(i) What is substance B?
(ii) What is the purpose of B?
(iii) Why is iron[III] chloride to be stored in a closed container?
(iv) Write the equation for the reaction between iron and chlorine.


Important Questions on Acids, Bases and Salts
Select the correct answer from A, B, C, D and E
A. Nitroso iron [II] sulphate
B. Iron [III] chloride
C. Chromium sulphate
D. Lead chloride
E. Sodium chloride
(i) A deliquescent compound
(ii) A compound soluble in hot water but insoluble in cold water.
(iii) A compound which in the aqueous solution state, is neutral in nature.
Give equations for the following conversions A to E-
For the preparation of the following salt- give a balanced equation.
Copper [II] sulphate from copper [II] oxide.
For the preparation of the following salt- give a balanced equation.
Iron [III] chloride from the metal ion.
For the preparation of the following salt- give a balanced equation.
Potassium sulphate from solution.
For the preparation of the following salt- give a balanced equation.
Lead [II] chloride from lead carbonate (give two equations).
