HARD
Earn 100
The diagram of the apparatus given to show that the acid solution in water conducts electricity.
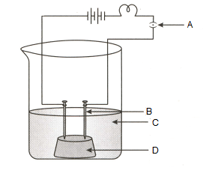
Identify the names of C and D from the given diagram:

(a)Switch
(b)Beaker
(c)Dilute solution
(d)Rubber cork
50% studentsanswered this correctly
Important Questions on Acids, Bases and Salts
MEDIUM
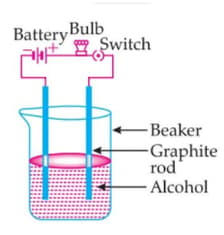
The bulb is not glowing in this experimental setup. Because:
MEDIUM
MEDIUM
MEDIUM
What is an alkali? Do basic solutions also have (aq) ions? If yes, then why are these basic?
HARD
Differentiate between the following pair based on the information given in the bracket.
- Acid and Alkali (formation of the type of ions)
EASY
EASY
MEDIUM
EASY
Which of the following is a strong acid?
EASY
MEDIUM
EASY
EASY
MEDIUM
Four solutions A, B, C, and D have pH 2, 6. 7, and 13 respectively:
Which solution will have the highest number of hydronium ions?
EASY
Give the appropriate term defined by the statement given below:
The substance that releases hydronium ion as the only positive ion when dissolved in water
MEDIUM
HARD
MEDIUM
MEDIUM

