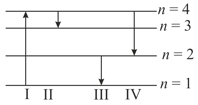
The diagram shown the energy levels for an electron in a certain atom. Which transition shown represents the emission of a photon with the most energy?


Important Questions on Atomic Physics
In problems involving electromagnetism it is often convenient and informative to express answers in terms of a constant, , which is a combination of the Coulomb constant, , the charge of the electron, , and being Planck's constant. For instance, the lowest energy that a hydrogen atom can have is given by , where is the mass of the electron and is the speed of light. Which of the following is the correct expression for ?
(HINT: non-relativistic kinetic energy is , where is speed.)
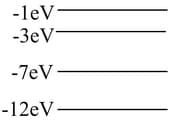
The energy level diagram is for a hypothetical atom. A gas of these atoms initially in the ground state is irradiated with photons having a continuous range of energies between and electron volts. One would) expect photons of which of the following energies to be emitted from the gas?

The diagram to the right shows the lowest four energy levels for an electron in a hypothetical atom. The electron is excited to the level of the atom and transitions to the lowest energy state by emitting only two photons. Which of the following energies could not belong to either of the photons?