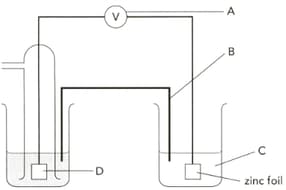
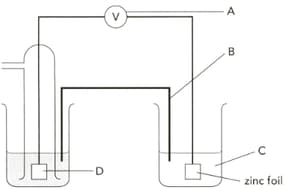
The diagram shows an electrochemical cell designed to find the standard electrode potential of zinc. Name the apparatus labelled "A" and give a characteristic it should have.


Important Questions on Electrochemistry
The diagram shows an electrochemical cell designed to find the standard electrode potential of zinc. Name the part "B" and give its two functions.
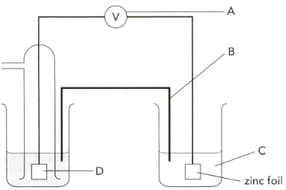
The diagram shows an electrochemical cell designed to find the standard electrode potential of zinc. Describe how part "B" can be prepared?
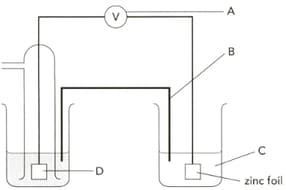
The diagram shows an electrochemical cell designed to find the standard electrode potential of zinc. Name"C" .
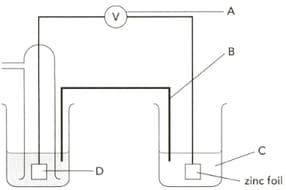
The diagram shows an electrochemical cell designed to find the standard electrode potential of zinc. Name part "D" and give its two functions.
The diagram shows an electrochemical cell involving two metal / metal ion system.
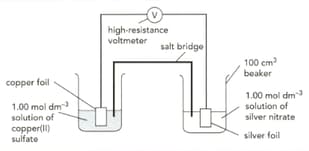
The standard electrode potentials for the half-cells are:
Calculate a value for the cell voltage. Show your working.
The diagram shows an electrochemical cell involving two metal / metal ion system.
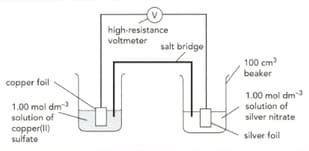
The standard electrode potentials for the half-cells are:
Write the balanced ionic equation for the overall cell reaction.
The diagram shows an electrochemical cell involving two metal / metal ion system.
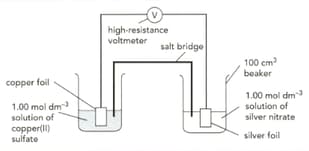
The standard electrode potentials for the half-cells are:
In this reaction, name the substance that is oxidised. Explain your answer.
