The diagram shows the structures of graphite and diamond.
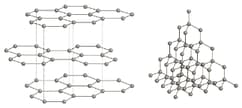
Use the diagrams and your knowledge of structure and bonding to answer the following question.
Explain why diamond is used on the tips of high-speed drills.

Explain why diamond is used on the tips of high-speed drills.

Important Questions on States of Matter
Describe a sodium chloride lattice.
Crystals of sodium chloride have a lattice structure.
Explain the following property of sodium chloride:
Sodium chloride has a high melting point.
Explain the following property of sodium chloride:
Sodium chloride conducts electricity when molten but not when solid.
Explain the following property of sodium chloride:
Sodium chloride is hard but brittle.
The diagram shows some allotropes of carbon.
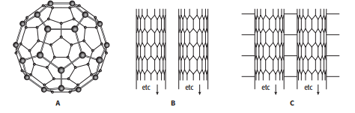
Explain in terms of structure and bonding why structure A is gaseous at 800 °C, but diamond is not.
The diagram shows some allotropes of carbon.
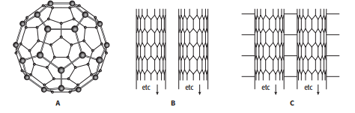
Structure B shows an allotrope of carbon in the form of tubes.
Describe the similarities and differences between structure B and graphite.
The diagram shows some allotropes of carbon.
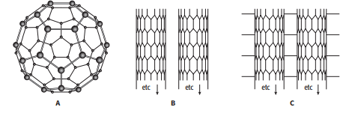
Structure C is stronger than structure B when a force is applied in the same direction as the long axis of the tube.
Explain why structure C is stronger.
