The diagram shows three of the energy levels in an isolated hydrogen atom. The lowest energy level is known as the ground state.
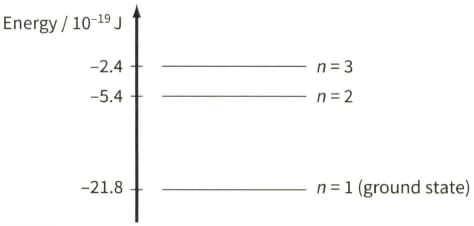
Calculate the wavelength of electromagnetic radiation emitted when an electron makes a jump between energy levels $n=3$ and $n=2$.


Important Questions on Quantum Physics
The diagram shows three of the energy levels in an isolated hydrogen atom. The lowest energy level is known as the ground state.
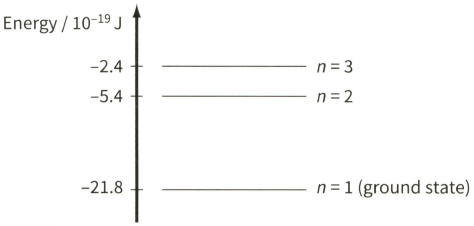
In the diagram, each energy level is labelled with its 'principal quantum number' $n$. Use the energy level diagram to show that the energy $E$ of an energy level is inversely proportional to $n^{2} .$
Explain how the photoelectric effect gives evidence for the wave-particle duality of electromagnetic radiation.
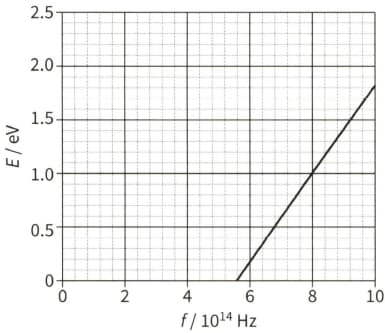
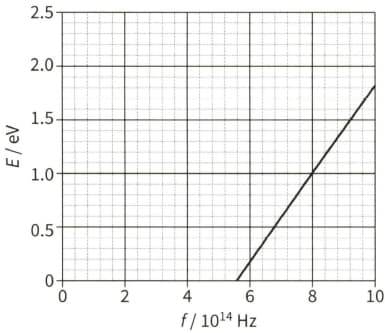
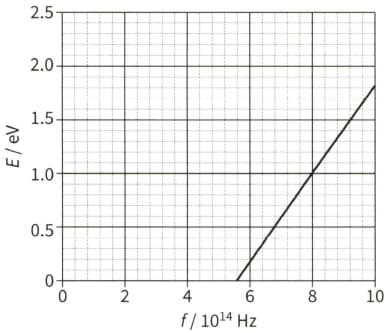
State what is meant by the de Broglie wavelength of an electron.
The diagram shows the principles of an electron tube used to demonstrate electron diffraction.
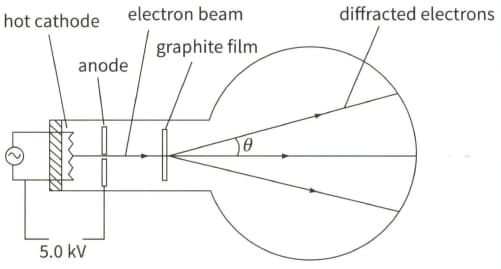
Calculate the kinetic energy $E$ (in joules) of the electrons incident on the graphite film.
