EASY
Earn 100
The dipole moment of and are 1.5 D, 0.5 D and 0 D respectively. The possible shapes of molecules may be (consider C has no Lone pair) (A, B and C are are more electronegative than X)
(a)Pyramidal, T-shape, Trigonal planar respectively.
(b)T-shape, Pyramidal, Square planar respectively.
(c)T-shape, Pyramidal, Trigonal planar respectively.
(d)Pyramidal, T-shape, Square planar respectively.
50% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
EASY
MEDIUM
EASY
EASY
EASY
MEDIUM
HARD
HARD
EASY
EASY
EASY
HARD
EASY
EASY
EASY
HARD
EASY
MEDIUM
HARD
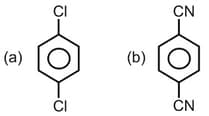
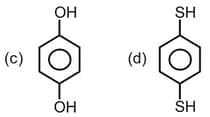
EASY

