EASY
Earn 100
The dipole moments of diatomic molecules AB and CD are 10.41D and 10.27D. respectively while their bond distances are 2.82 and 2.67 , respectively. This indicates that
(a)Bonding is 100% ionic in both the molecules
(b)AB has more ionic bond character than CD
(c)AB has lesser ionic bond character than CD
(d)Bonding is nearly covalent in both the molecules
50% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
EASY
HARD
EASY
EASY
EASY
EASY
EASY
EASY
EASY
MEDIUM
EASY
EASY
EASY
MEDIUM
HARD
MEDIUM
How many of the following molecules are with non-zero net dipole moment,
EASY
HARD
HARD
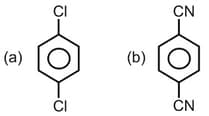
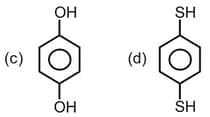
EASY

