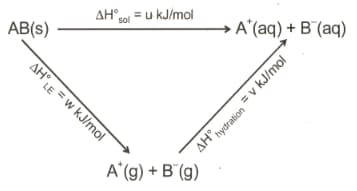
MEDIUM
NEET
IMPORTANT
Earn 100
The dissociation energy of is and that of () is . The bond energy
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermodynamics
MEDIUM
NEET
IMPORTANT
The enthalpy of reaction,
If the bond energies of and bonds are respectively
MEDIUM
NEET
IMPORTANT
Using bond energy data, calculate heat of formation of isoprene
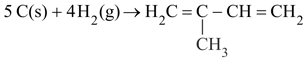
Given and respectively. as
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
If the enthalpy change (i.e. ) for the given reaction is at then the value of is
MEDIUM
NEET
IMPORTANT
The heat of combustion of ethene from the given data is
Bond Bond Energy
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
Consider the following
The correct relation between is