MEDIUM
MHT-CET
IMPORTANT
Earn 100
The distance between an octahedral and tetrahedral void in FCC lattice would be:
(a)
(b)
(c)
(d)
23.08% studentsanswered this correctly

Important Questions on Solid State
MEDIUM
MHT-CET
IMPORTANT
has type structure with density . If the ionic radius of is , determine the ionic radius of (Molar mass of )
MEDIUM
MHT-CET
IMPORTANT
Caesium chloride crystal's interionic distance will be:
MEDIUM
MHT-CET
IMPORTANT
In a lattice, what fraction of edge is not covered by atoms?
MEDIUM
MHT-CET
IMPORTANT
An atomic solid crystallizes in a body-centred cubic lattice and the surface of the atoms at the adjacent corner are separated by . If the atomic weight of is , the density of the solid, is nearly
MEDIUM
MHT-CET
IMPORTANT
For the structure given below, the site marked as is a:
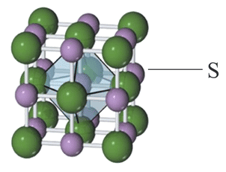
MEDIUM
MHT-CET
IMPORTANT
An element crystallizes in a '' lattice. Nearest neighbours and next-nearest neighbours of the element are, respectively:
MEDIUM
MHT-CET
IMPORTANT
The radius of divalent cation and of divalent anion is and respectively. Thus, has:
MEDIUM
MHT-CET
IMPORTANT
crystallizes in a type lattice with the edge length of unit cell equal to If the radius of the ion is , then calculate the radius of .
