MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
The of the following cell
at is The electrical energy which can be produced is :-
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Electrochemistry
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
is produced by the electrolytic reduction of ion in presence of ion is Calculate the current required to have a rate production of per hour of
[Atomic weight of
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
Consider the following Galvanic cell as shown in the figure:
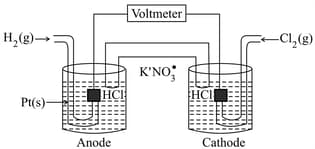
By what value will the cell voltage change when concentration of ions in anodic and cathodic compartments are both increased by a factor of at .
MEDIUM
JEE Main/Advance
IMPORTANT
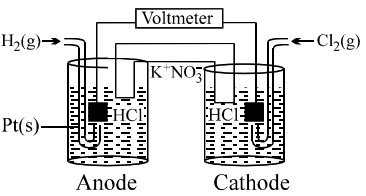
By what value the cell voltage change when concentration of ions in anodic and cathodic compartment both increased by factor of 10 at 298 K.
MEDIUM
JEE Main/Advance
IMPORTANT
