EASY
JEE Main
IMPORTANT
Earn 100
The efficiency of a Carnot engine working between and is
(a)
(b)
(c)
(d)
(e)
50% studentsanswered this correctly

Important Questions on The First Law of Thermodynamics
EASY
JEE Main
IMPORTANT
The efficiency of Carnot heat engine is , when the temperature of the source is and that of sink is . The efficiency of another Carnot heat engine is also . The temperatures of source and sink of the second engine respectively are
MEDIUM
JEE Main
IMPORTANT
A Carnot engine having an efficiency of is being used as a refrigerator. If the work done on the refrigerator is , then the amount of heat absorbed from the reservoir at lower temperature is,
MEDIUM
JEE Main
IMPORTANT
A thermodynamic cycle is shown on a diagram.
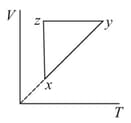
The - diagram that best describes this cycle is (diagrams are schematic and not to scale),
HARD
JEE Main
IMPORTANT
A litre of dry air at STP expands adiabatically to a volume of . If , the work done by air is [take air to be an ideal gas],
HARD
JEE Main
IMPORTANT
Under an adiabatic process, the volume of an ideal gas gets doubled. Consequently, the mean collision time between the gas molecule changes from to . If for this gas, then a good estimate for is given by,
MEDIUM
JEE Main
IMPORTANT
In a process, temperature and volume of one mole of an ideal mono-atomic gas are varied according to the relation, , where is a constant. In this process, the temperature of the gas is increased by . The amount of heat absorbed by gas is (where is gas constant),
EASY
JEE Main
IMPORTANT
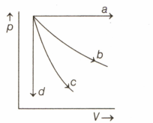
The given diagram shows four processes, i.e., isochoric, isobaric, isothermal and adiabatic. The correct assignment of the processes in the same order is given by,
EASY
JEE Main
IMPORTANT
An ideal mono-atomic gas at expands adiabatically to twice its volume. The final temperature of gas is,
