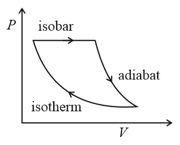
The efficiency of the cycle shown below in the figure (consisting of one isobar, one adiabat and one isotherm) is The ratio, between the highest and lowest temperatures attained in this cycle obeys (the working substance is a ideal gas):-


Important Questions on Thermodynamics
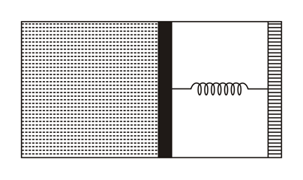
An ideal monoatomic gas is confined in a horizontal cylinder by a spring loaded piston (as shown in the figure). Initially the gas is at temperature , pressure and volume and the spring is in its relaxed state. The gas is then heated very slowly to temperature , pressure and volume . During this process, the piston moves out by a distance . Ignoring the friction between the piston and the cylinder, the correct statement(s) is(are)
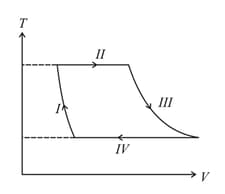
One mole of a monatomic ideal gas undergoes a cyclic process as shown in the figure (where is the volume and is the temperature). Which of the statements below is (are) true ?
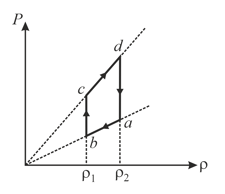
An ideal gas undergoes a cyclic process which is shown by pressure-density curve.