HARD
Earn 100
The electrochemical cell shown below is a concentration cell.
The emf of the cell depends on the difference in concentrations of ions at the two electrodes. The emf of the cell at is
The solubility product of based on the information available for the given concentration cell is?
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Ionic Equilibrium
MEDIUM
MEDIUM
HARD
MEDIUM
EASY
MEDIUM
HARD
MEDIUM
HARD
HARD
MEDIUM
MEDIUM
[Solubility product for ]
MEDIUM
Which of the following choices is correct for a mixture of and
MEDIUM
MEDIUM
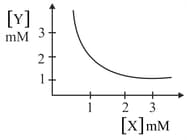
The stoichiometry and solubility product of a salt with the solubility curve given below is, respectively:
EASY
EASY
MEDIUM
HARD
EASY

