HARD
10th CBSE
IMPORTANT
Earn 100
The energy diagram shows the energy levels of reactants and products of a particular reaction.
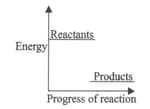
Which of the following processes can be related to the given diagram?

(a)Ethylene gas burns in oxygen to form carbon dioxide and water along with the evolution of heat.
(b)When solid mercury oxide is heated liquid mercury and oxygen gas are produced.
(c)Hydrogen gas combines with chlorine gas in the presence of light to form hydrogen chloride gas.
(d)Potassium chlorate decomposes in the presence of heat to form potassium chloride and oxygen.
50% studentsanswered this correctly

Important Questions on Chemical Reactions And Equations
EASY
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
| Chemical reactions | Observable features | ||
| Change in temperature | Magnesium reacting with dilute sulphuric acid | ||
| Evolution of gas | Potassium iodide with lead nitrate | ||
| Formation of precipitate | Sulphur dioxide gas reacting with acidified potassium dichromate solution | ||
| Change in colour | Zinc granules reacting with dilute sulphuric acid |
EASY
10th CBSE
IMPORTANT
A science teacher wrote the following statements about rancidity:
When fats and oils are reduced, they become rancid.
In chips packet, rancidity is prevented by oxygen.
Rancidity is prevented by adding antioxidants.
Select the correct option.
EASY
10th CBSE
IMPORTANT
Which on of the following is an example of a redox reaction?
MEDIUM
10th CBSE
IMPORTANT
Classify each of the following reactions:
EASY
10th CBSE
IMPORTANT
and are three metals that undergo chemical reactions as follows:
Observe the reactions and arrange the metals in the increasing order of their reactivity.
