MEDIUM
11th CBSE
IMPORTANT
Earn 100
The energy of an electron in the first Bohr orbit of atom is . The possible energy value (s) of the excited state(s) for electron in Bohr orbits of hydrogen is(are)
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Structure of Atom
MEDIUM
11th CBSE
IMPORTANT
EASY
11th CBSE
IMPORTANT
MEDIUM
11th CBSE
IMPORTANT
EASY
11th CBSE
IMPORTANT
HARD
11th CBSE
IMPORTANT
| Column I | Column II | ||
| A | Light has dual character | p | de Broglie |
| B | Electrons have dual character | q | Einstein |
| C |
has electronic configuration and |
r | Aufbau |
| D | orbital is filled first than | s | Hunds |
| E | Basis of Quantum mechanics | t | Schrodinger |
HARD
11th CBSE
IMPORTANT
| Column 1 | Column 2 | Column 3 |
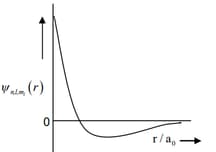
| (I) orbital | (i) | 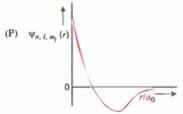 |
| (II) orbital | (ii) One radial node | (Q) Probability density at nucleus |
| (III) orbital | (iii) | (R) Probability density is maximum at nucleus |
| (IV) orbital | (iv) -plane is a nodal plane | (S) Energy needed to excite electron from state to state is times the energy needed to excite electron from state to state |
For ion, the only INCORRECT combination is
HARD
11th CBSE
IMPORTANT
| Column 1 | Column 2 | Column 3 |
| (I) orbital | (i) | 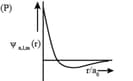 |
| (II) orbital | (ii) One radial node | (Q) Probability density at nucleus |
| (III) orbital | (iii) | (R) Probability density is maximum at nucleus |
| (IV) orbital | (iv) -plane is a nodal plane | (S) Energy needed to excite electron from state to state is times the energy needed to excite electron from state to state |
For the given orbital in column, the only CORRECT combination for any hydrogen-like species is
HARD
11th CBSE
IMPORTANT
| Column 1 | Column 2 | Column 3 |
| (I) orbital | (i) |
(P)
|
| (II) orbital | (ii) One radial node | (Q) Probability density at nucleus |
| (III) orbital | (iii) | (R) Probability density is maximum at nucleus |
| (IV) orbital | (iv) -plane is a nodal plane |
(S) Energy needed to excite electron from |
For the hydrogen atom, the only CORRECT combination is