MEDIUM
NEET
IMPORTANT
Earn 100
The enthalpies of combustion of carbon and carbon monoxide are , respectively. The enthalpy of formation of carbon monoxide per mole is:
(a)
(b)
(c)
(d)
33.33% studentsanswered this correctly

Important Questions on Thermodynamics
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
For a particular reaction and This reaction is
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
Look at the following diagram:
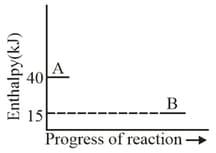
The enthalpy change for the reaction will be
