EASY
Diploma
IMPORTANT
Earn 100
The enthalpy of combustion of methanol can also be determined experimentally in a school laboratory. A burner containing methanol was weighed and used to heat water in a test tube as illustrated in figure.
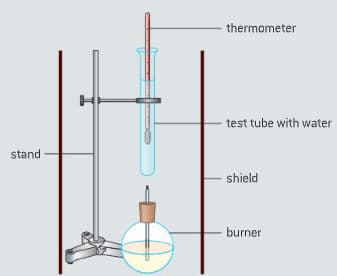
The data shown in table were collected.
Initial mass of burner and methanol /
Final mass of burner and methanol /
Mass of water in test tube /
Initial temperature of water /
Final temperature of water
The data booklet value for the enthalpy of combustion of methanol is . Suggest why this value differs from the values calculated experimentally.


Important Questions on Energetics and Thermochemistry
EASY
Diploma
IMPORTANT
Scientists perform experiments and process the raw data to enable us to draw conclusions. We compare experimental and theoretical values. What criteria do we use when making these comparisons? Are our judgments subjective or objective? When analysing and appraising experimental limitations and making theoretical assumptions, which of the ways of knowing are we utilising?
EASY
Diploma
IMPORTANT
Write equation to describe the standard enthalpy change of formation for propane and state the enthalpy value by referring to the data booklet.
EASY
Diploma
IMPORTANT
Write equation to describe the standard enthalpy change of formation for chloromethane.
EASY
Diploma
IMPORTANT
Write equation to describe the standard enthalpy change of formation for ethanol.
EASY
Diploma
IMPORTANT
Write equation to describe the standard enthalpy change of formation for benzoic acid.
EASY
Diploma
IMPORTANT
Write equation to describe the standard enthalpy change of formation for carbon monoxide.
EASY
Diploma
IMPORTANT
Write equation to describe the standard enthalpy change of formation for methylamine.
EASY
Diploma
IMPORTANT
Write equation to describe the standard enthalpy change of combustion for octane, .
