HARD
JEE Main/Advance
IMPORTANT
Earn 100
The enthalpy of combustion of propane gas in terms of given data is :
Bond energy
Resonance energy of is and is .
(a)
(b)
(c)
(d)
77.78% studentsanswered this correctly

Important Questions on Thermodynamics
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
The heat of formation of in tents of and is:
HARD
JEE Main/Advance
IMPORTANT
Ethanol can undergo decomposition to form two sets of products.
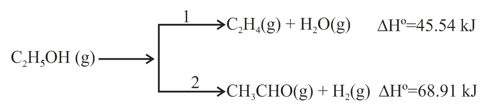
If the molar ratio of to is in a set of product gases, then the energy involved in the decomposition of mole of ethanol is:
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
From given following equations and values at determine the enthalpy of reaction (in
?
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
