MEDIUM
Earn 100
The equilibrium that exists in aqueous solution, if dil is added at constant temperature, then
(a)The equilibrium constant will increase
(b)The equilibrium constant will decrease
(c)Concentration of will decrease
(d)Concentration of will increase
50% studentsanswered this correctly
Important Questions on Equilibrium
HARD
EASY
HARD
MEDIUM
HARD
MEDIUM
MEDIUM
MEDIUM
EASY
HARD
MEDIUM
MEDIUM
[Solubility product for ]
MEDIUM
Which of the following choices is correct for a mixture of and
MEDIUM
MEDIUM
The stoichiometry and solubility product of a salt with the solubility curve given below is, respectively:
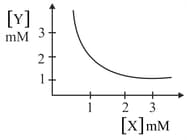
EASY
EASY
MEDIUM
HARD
MEDIUM

