HARD
Earn 100
The equivalent conductance () of an aqueous solution of a strong electrolyte of concentration is given by , where is a constant and is the concentration of the electrolyte. The specific conductance (with ) of and salt solutions (aqueous) are plotted against . The correct figure is: (The plots are not drawn to scale.)
(a)
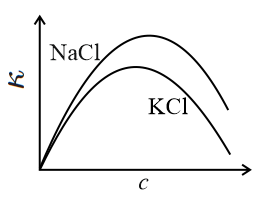
(b)
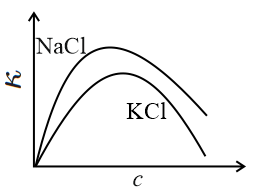
(c)
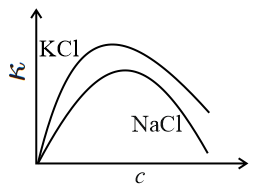
(d)
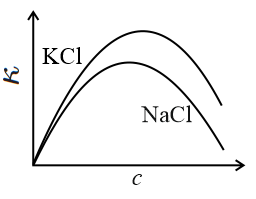
40% studentsanswered this correctly
Important Questions on Electrochemistry
EASY
EASY
EASY
Resistivity
HARD
MEDIUM
MEDIUM
MEDIUM
EASY
EASY
EASY
MEDIUM
EASY
EASY
[Given: equivalent conductance at infinite dilution of and ]
MEDIUM
EASY
MEDIUM
Conductivity of dilute hydrochloric acid is greater than that of acetic acid.
MEDIUM
MEDIUM
MEDIUM
EASY
Formic acid,
Acetic acid,
Benzoic acid.

