MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
The equivalent conductance of a solution at is Resistance of solution contained in the cell is cell constant is:
(a)
(b)
(c)
(d)
37.5% studentsanswered this correctly

Important Questions on Electrochemistry
HARD
JEE Main/Advance
IMPORTANT
For an (aq.) solution, which of the following quantities go to zero as concentration goes to.
(Assume the solvent's contribution to conductivity has been subtracted off).
MEDIUM
JEE Main/Advance
IMPORTANT
A graph of molar conductivity of three electrolytes and is plotted against
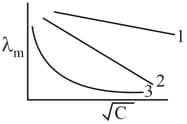
Which of the following options is correct?
MEDIUM
JEE Main/Advance
IMPORTANT
molar solution filled in different conductivity cell. Order of equivalent conductance of solution is:
| Cell- | Cell- | Cell- | |
| Equivalents : |
conductance Area of cross-section, distance between two electrodes.
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
