MEDIUM
Physics
IMPORTANT
Earn 100
The expansion of an ideal gas of mass m at a constant pressure P is given by the straight line D. Then the expansion of the same ideal gas of mass 2m at a pressure P/2 is given by the straight line
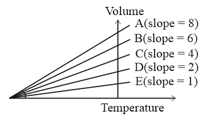
(a)E
(b)C
(c)B
(d)A
50% studentsanswered this correctly

Important Questions on Kinetic Theory of Gases
EASY
Physics
IMPORTANT
Two gases occupy two containers and ; the gas in , of volume , exerts a pressure of and that in , of volume , exerts a pressure . The two containers are joined by a tube of negligible volume and the gases are allowed to intermingle. Then if the temperature remains constant, the final pressure in the containers will be (in )
EASY
Physics
IMPORTANT
A closed vessel contains 8g of oxygen and 7 g of nitrogen. The total pressure is 10 atm at a given temperature. If now oxygen is absorbed by introducing a suitable absorbent, the pressure of the remaining gas in atm will be
EASY
Physics
IMPORTANT
Energy of all molecules of a monatomic gas having a volume V and pressure P is 3/2 PV. The total translational kinetic energy of all molecules of a diatomic gas at the same volume and pressure is
MEDIUM
Physics
IMPORTANT
A gas mixture consists of moles of oxygen and moles of argon at temperature . Neglecting all vibrational modes, the total internal energy of the system is
MEDIUM
Physics
IMPORTANT
A stationary cylinder of oxygen used in a hospital has the following characteristics at room temperature 300 K, gauge pressure 1.38 x 107 Pa, volume 16 L. If the flow area, measured at atmospheric pressure, is constant at 2.4 L/min, the cylinder will last for nearly
EASY
Physics
IMPORTANT
Internal energy of mol of hydrogen of temperature is equal to the internal energy of of helium at temperature . The ratio is
EASY
Physics
IMPORTANT
If of an ideal monatomic gas at temperature is mixed with of another ideal monatomic gas at temperature , then the temperature of the mixture is___
EASY
Physics
IMPORTANT
If is the atmospheric pressure in the previous problem, find the percentage increase in tension of the string after heating:
