HARD
Earn 100
The experimental value of the dipole moment of is . The length of the bond is . The percentage of ionic character in is:
[Given: ]
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
EASY
HARD
EASY
EASY
EASY
EASY
EASY
EASY
EASY
EASY
EASY
HARD
MEDIUM
HARD
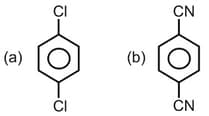
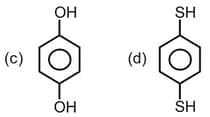
HARD

