MEDIUM
NEET
IMPORTANT
Earn 100
The figure indicates the energy level diagram for the origin of six spectral lines in the emission spectrum(e.g. line number arises from the transition from level to ) which of the following spectral lines will not occur in the absorption spectrum: -
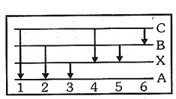

(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Structure of Atom
EASY
NEET
IMPORTANT
A certain electronic transition from an excited state to the ground state in a sample of atoms gives rise to maximum of three lines in the ultraviolet region of the spectrum. How many lines does this transition produce in the infrared region of the spectrum?
HARD
NEET
IMPORTANT
Four lowest energy levels of atom are shown in the figure. The number of emission lines could be

MEDIUM
NEET
IMPORTANT
The number of absorption lines in the following figure could be :-

MEDIUM
NEET
IMPORTANT
If energy is supplied to the atom, the number of spectral lines emitted is equal to:
MEDIUM
NEET
IMPORTANT
What is the de-Broglie's wavelength associated with the electron in its third orbit?
MEDIUM
NEET
IMPORTANT
Number of waves in fourth orbit : -
MEDIUM
NEET
IMPORTANT
What is the ratio of the de-Broglie wavelengths for electrons accelerated through volts and volts?
MEDIUM
NEET
IMPORTANT
For a valid Bohr orbit, its circumference should be
