MEDIUM
TS EAMCET
IMPORTANT
Earn 100
The figure represents vs relation for and gases under identical conditions. Which curve, shown in the figure, represents gas?
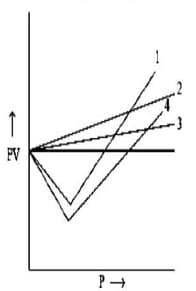

(a)
(b)
(c)
(d)
17.65% studentsanswered this correctly

Important Questions on States of Matter : Gases and Liquids
EASY
TS EAMCET
IMPORTANT
One mole of an ideal gas occupies at . What is the pressure of the gas?
MEDIUM
TS EAMCET
IMPORTANT
grams of a gas at and pressure occupies a volume of . The gas can be
EASY
TS EAMCET
IMPORTANT
Which gas has a density of at and pressure?
MEDIUM
TS EAMCET
IMPORTANT
The kinetic energy in of mole of at is:
MEDIUM
TS EAMCET
IMPORTANT
The most probable velocity of a gas at is equal to the RMS velocity of gas at The gas is
MEDIUM
TS EAMCET
IMPORTANT
How many times the volume of a diatomic gas should be increased reversibly and adiabatically in order to reduce its RMS velocity to half of its intial value.
MEDIUM
TS EAMCET
IMPORTANT
Diffusion of and occurs under similar conditions, then the ratio of their rates of diffusion is
MEDIUM
TS EAMCET
IMPORTANT
The ratio of rates of diffusion of oxygen and an unknown gas is . The unknown gas is
