MEDIUM
NEET
IMPORTANT
Earn 100
The figure shows a graph between and , where is the area enclosed by the orbit in a hydrogen-like atom. The correct curve which represents this relation is,
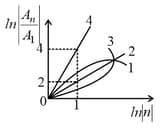

(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Atoms
MEDIUM
NEET
IMPORTANT
Consider a hydrogen-like atom whose energy in excited state is given by . When this excited atom makes a transition from the excited to the ground state, the most energetic photons have energy and least energetic photons have energy The atomic number of the atom is
EASY
NEET
IMPORTANT
An electron revolves around a nucleus of charge . In order to excite the electron from the state to , the energy required is . Then atomic number is
EASY
NEET
IMPORTANT
A hydrogen atom rises from its state to the state by absorbing energy. If the potential energy of the atom in the state be , then potential energy in the state will be
EASY
NEET
IMPORTANT
The radius of first orbit of hydrogen atom is . The radius of its fourth orbit will be
EASY
NEET
IMPORTANT
The binding energy of an electron with in the H-atom is
EASY
NEET
IMPORTANT
The lifetime of an electron in the state in the hydrogen atom is about
HARD
NEET
IMPORTANT
A particular hydrogen-like atom has its ground state binding energy . It is in ground state. Then select the incorrect option.
MEDIUM
NEET
IMPORTANT
In the hydrogen atom, if the reference level of potential energy is assumed to be zero at the ground state level, then choose the incorrect statement.
