HARD
Earn 100
The figure shows electronic wave function for a hydrogen atom.
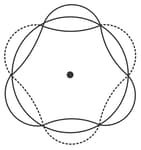

(a)The quantum number of this state is .
(b)The wavelength of this electron is ( is radius of ground state).
(c)It can go to ground state by emitting different photons.
(d)On DE excitation it emits at least one line in infra-red region of spectrum.
16.67% studentsanswered this correctly
Important Questions on Dual Nature of Radiation and Matter
EASY
MEDIUM
MEDIUM
EASY
( being velocity of light)
MEDIUM
The de-Broglie wavelength associated with the electron in the level is:
EASY
MEDIUM
MEDIUM
HARD
EASY
EASY
EASY
EASY
EASY
EASY
HARD
MEDIUM
HARD
EASY
EASY
| List-I | List-II | ||
| A | Franck-Hertz Experiment | (i) | Particle nature of light |
| B | Photo-electric experiment | (ii) | Discrete energy levels of the atom |
| C | Davison-Germer Experiment | (iii) | Wave nature of electron |
| (iv) | Structure of atom |

