The first electron affinity is the energy change when one mole of gaseous atoms gains a mole of electrons. The table below shows the first electron affinity for selected atoms.
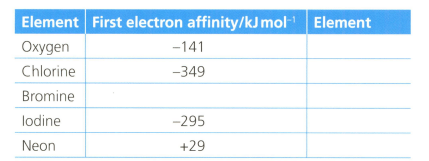
The first electron affinity of oxygen is .
In terms of electrostatics, oxygen has a lower first electron affinity than chlorine.
Explain why neon has a positive value of electron affinity.


Important Questions on Why do Electrons Matter?
A hydrogen atom has the main energy levels (shells) shown in the diagram below. refers to the shell number. This is the Bohr model of the atom.
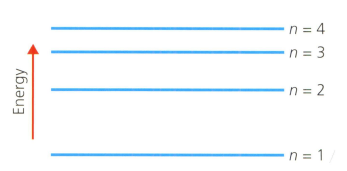
Describe the electron transition (movement) within the hydrogen atom using the shell numbers indicated above, that will release the most energy.
A hydrogen atom has the main energy levels (shells) shown in the diagram below. refers to the shell number. This is the Bohr model of the atom.
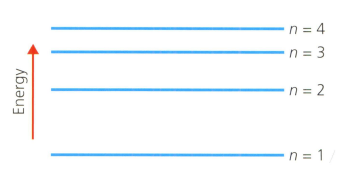
Describe the electron transition (movement) within the hydrogen atom using the shell numbers indicated above, that will absorb the least energy.
A hydrogen atom has the main energy levels (shells) shown in the diagram below. refers to the shell number. This is the Bohr model of the atom.
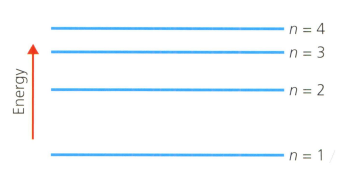
List these electron transitions in order of increasing wavelength of light absorbed or emitted:
- to
- to
- to
- to
A hydrogen atom has the main energy levels (shells) shown in the diagram below. refers to the shell number. This is the Bohr model of the atom.
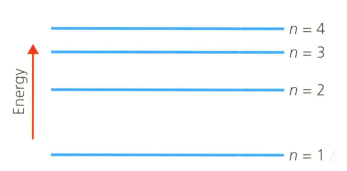
Explain why a ladder is a good analogy or model for visualising the energy levels of atoms (and molecules), and what are the limitations of the analogy.
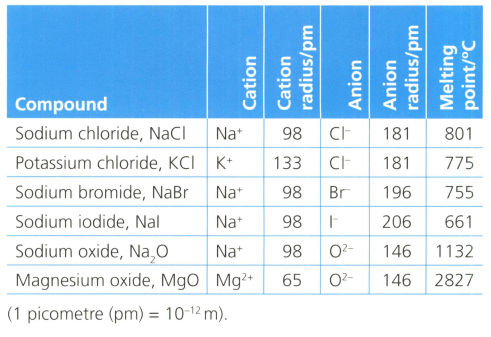
Analyse and evaluate the data about ionic compounds that are listed in the table below.
Identify and make scientifically supported judgments to explain any relationships between the size (radius) of ions present, and the predicted properties of their compounds. Your explanation should refer to your knowledge of ions, ionic bonding and the behaviour of electric charges. State any assumptions in your explanation.