EASY
Earn 100
The first reliable measurement on the properties of gases was made by scientist _____.
(a)Dalton
(b)Robert Brown
(c)Robert Boyle
(d)Jacques Charles'
50% studentsanswered this correctly
Important Questions on States of Matter
MEDIUM
EASY
For the given isotherms, which of the following is correct for
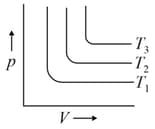
MEDIUM
EASY
EASY
EASY
MEDIUM
The volume of gas is twice than that of gas . The compressibility factor of gas is thrice than that of gas at same temperature. What are the pressures of the gases for equal number of moles?
MEDIUM
EASY
MEDIUM
MEDIUM
[Gas constant, ]
EASY
EASY
EASY
At constant pressure, the volume of a fixed mass of a gas varies as a function on temperature as shown in the graph
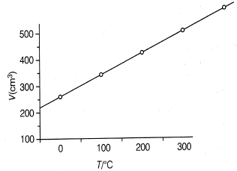
The volume of the gas at is larger than that at by a factor of
EASY
EASY
HARD
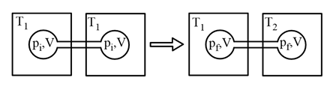
MEDIUM
MEDIUM
EASY

