MEDIUM
12th ICSE
IMPORTANT
Earn 100
The following curve is obtained when molar conductivity, is plotted against the square root of concentration, along and -axis respectively for the two electrolytes and . Account for the increase in for the electrolytes and with dilution.
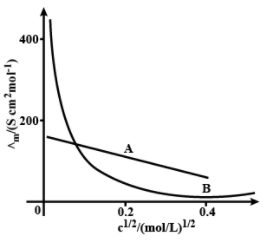


Important Questions on Electrochemistry
MEDIUM
12th ICSE
IMPORTANT
The following curve is obtained when molar conductivity, is plotted against the square root of concentration, along and -axis respectively for the two electrolytes and . Determine for these electrolytes.
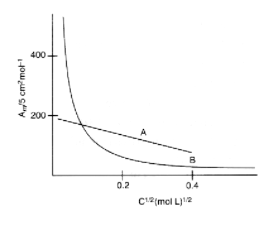
HARD
12th ICSE
IMPORTANT
HARD
12th ICSE
IMPORTANT
MEDIUM
12th ICSE
IMPORTANT
HARD
12th ICSE
IMPORTANT
EASY
12th ICSE
IMPORTANT
EASY
12th ICSE
IMPORTANT
MEDIUM
12th ICSE
IMPORTANT
How many moles of will be produced by electrolysing solution with a current of for hours?
