MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
The following data were obtained in an experiment on inversion of cane sugar; (a first order kinetics)
Time
After a long time
Total angle of rotation (degree)
The rate constant (in ) is
(a)
(b)
(c)
(d)
66.67% studentsanswered this correctly

Important Questions on Chemical Kinetics
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
The decomposition of in chloroform was followed by measuring the volume of O2 gas evolved : ()() + . The maximum volume of gasobtained was. In 500 minutes, of were evolved. The first order rate constant for the disappearance of is :
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
For a hypothetical elementary reaction.
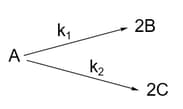
where
Initially, only moles of are present. The total number of moles of and at the end of of reaction are:
HARD
JEE Main/Advance
IMPORTANT
For the following parallel chain reaction 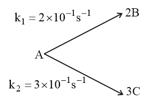 , if the sum of the concentration of and at any time is . What will be and respectively?
, if the sum of the concentration of and at any time is . What will be and respectively?
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
