EASY
TS EAMCET
IMPORTANT
Earn 100
The following figure shows a Carnot engine that works between temperatures and and drives a Carnot refrigeration that works between temperatures and The quantity will be
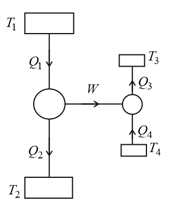
(a)
(b)
(c)
(d)
8.7% studentsanswered this correctly

Important Questions on Thermodynamics
HARD
TS EAMCET
IMPORTANT
A one mole of ideal gas goes through a process in which pressure varies with volume as , where is a constant. The maximum attainable temperature by the ideal gas during this process is (All quantities are in units and is gas constant)
HARD
TS EAMCET
IMPORTANT
A diatomic gas of volume at pressure is compressed adiabatically to a volume The work done in this process is,
[Use ]
EASY
TS EAMCET
IMPORTANT
of water is heated from to What is the change in its internal energy? (Specific heat of water is )
MEDIUM
TS EAMCET
IMPORTANT
The diagram shown below indicates two paths along which a sample of gas can be taken from state to state . The energy equal to in the form of heat is required to be transferred if the Path- is chosen. How much energy in the form of heat should be transferred if Path- is chosen?
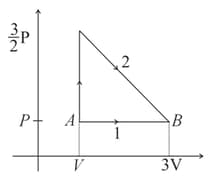
MEDIUM
TS EAMCET
IMPORTANT
The internal energy of the air, in a room of volume , at temperature and with outside pressure increasing linearly with time, varies as
MEDIUM
TS EAMCET
IMPORTANT
An air bubble rises from the bottom of a water tank of height . If the initial volume of the bubble is . What will be its volume as it reaches the surface. Assume that its temperature does not change.
[, density of water ]
MEDIUM
TS EAMCET
IMPORTANT
A tyre pumped to a pressure of atmosphere, suddenly bursts. If the temperature of air before expansion is then the air temperature after the tyre busts is
(Assume the expansion is adiabatic and adiabatic constant
EASY
TS EAMCET
IMPORTANT
An ideal gas undergoes an adiabatic process. If the pressure of the gas is reduced by then the volume is changed by (Given )
