The following figure shows the fractions of the four forms of a tribasic acid Which line represents
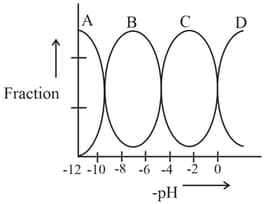

Important Questions on Equilibrium
aqueous solution of at is _________ (Nearest integer)
| Acid | Ionization constant, | ||
| A) | Formic acid | I) | |
| B) | Niacin | II) | |
| C) | Hypochlorous acid | III) | |
| D) | Hydrocyanic acid | IV) | |
The correct match is
moles of a weak acid is dissolved in of solution. The degree of dissociation of is __________ (Round off to the Nearest Integer). [Neglect volume change on adding and assume degree of dissociation ]
| List-1 Acid |
List-2 Ka(ionization constant) |
||
| A) | I) | ||
| B) | II) | ||
| C) | III) | ||
| D) | Niacin | IV) | |
| V) | |||
The correct match is
Given below are two statements: one is labelled as Assertion and the other is labelled as Reason .
Assertion :Phenolphthalein is a dependent indicator, remains colourless in acidic solution and gives pink colour in basic medium
Reason : Phenolphthalein is a weak acid. It doesn't dissociate in basic medium.
In the light of the above statements, choose the most appropriate answer from the options given below
The degree of dissociation of acetic acid in water is
( of acetic acid is )

