EASY
Earn 100
The following optical isomerism in octahedral complex with bidentate ligand can be represented as:
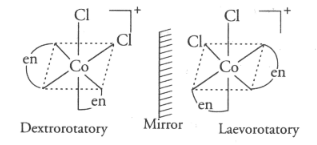

(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Coordination Compounds
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
HARD
Which of the following complex ions does not possess optical isomerism?
MEDIUM
MEDIUM
Here en = ethylene diamine
MEDIUM
EASY
MEDIUM
What is the reason for optical isomerism in co-ordination compounds? Explain by giving one example. Give IUPAC names of the following compounds: .
MEDIUM
HARD
HARD
What is optical isomerism? Draw the structure of optical isomers of .
MEDIUM
MEDIUM
HARD
EASY
MEDIUM
(ethanediamine)
EASY

