EASY
Earn 100
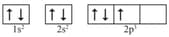
The following orbital diagram shows the electronic configuration of the nitrogen atom. Which rule does not support this?

Important Questions on Atomic Structure
EASY
EASY
EASY
EASY
EASY
(At. No. Z = )
MEDIUM
MEDIUM
EASY
EASY
EASY
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
EASY
MEDIUM

