HARD
NEET
IMPORTANT
Earn 100
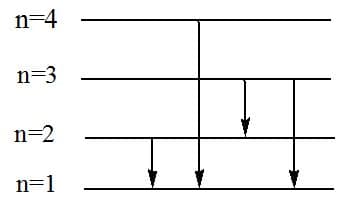
The frequency of radiation emitted when the electron falls from to in a hydrogen atom will be
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Structure of Atom
HARD
NEET
IMPORTANT
The kinetic energy of an electron in the second orbit of a hydrogen atom is
( is Bohr radius of the first orbit)
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
Suppose that a hypothetical atom gives a red, green, blue and violet fine spectrum . Which jump according to figure would give off the red spectral line.
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT