The fusion reaction that holds most promise for the generation of electricity is the fusion of tritium and deuterium .
The following equation shows the process:
Calculate:
(a) the change in mass in the reaction
(Mass of , Mass of , Mass of , Mass of )

Important Questions on Nuclear Physics
The fusion reaction that holds most promise for the generation of electricity is the fusion of tritium and deuterium .
The following equation shows the process:
Calculate:
(b) the energy released in the reaction
(Mass of , Mass of , Mass of , Mass of )
The fusion reaction that holds most promise for the generation of electricity is the fusion of tritium and deuterium . The following equation shows the process:
Calculate:
(c) the energy released if one mole of deuterium were reacted with one mole of tritium.
(Mass of , mass of , mass of mass of )
The initial activity a sample of 1 mole of radon-220 is .
Calculate:
(a) the decay constant for this isotope.
The initial activity a sample of 1 mole of radon-220 is .
Calculate:
(b) the half-life of the isotope.
The graph of count rate against time for a sample containing indium-116 is shown.
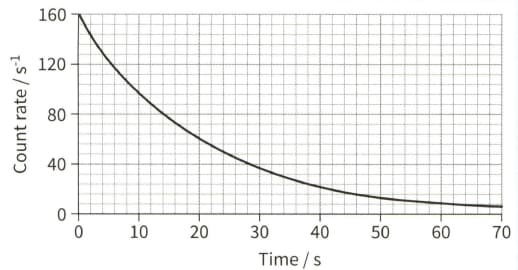
(a) Use the graph to determine the half-life of the isotope.
The graph of count rate against time for a sample containing indium-116 is shown.
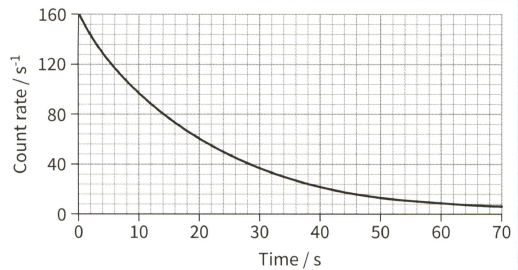
(b) Calculate the decay constant.
The proportions of different isotopes in rocks can be used to date the rocks. The half-life of uranium-238 is years. A sample has of the proportion of this isotope compared with newly formed rock.
(a) Calculate the decay constant in for this isotope of uranium.
The proportions of different isotopes in rocks can be used to date the rocks. The half-life of uranium-238 is years. A sample has of the proportion of this isotope compared with newly formed rock.
(b) Calculate the age of the rock in years.
